Should I use UN3373, UN2914 or UN2900 packaging to transport infectious samples?
The transport of Category A or Category B specimens, otherwise known as infectious substances, must comply with international requirements on packaging, labelling and documentation to ensure the safety of those handling the shipment along the supply chain. While Category B specimens do not pose as high a risk as Category A substances, it is still important to follow the labelling and packaging requirements for shipping.
In this article, we will cover specifications of UN3373, UN2914 or UN2900 packaging and labelling and which is the most appropriate for your shipment.
How do I know which samples are Category A or Category B?
The transport of infectious substances is governed by UN Regulations Division 6.2 which splits samples into two distinct categories, namely Category A and Category B.
Category A further divides samples into two sub-divisions namely UN2814 (affecting humans) and UN2900 (affecting animals only) and covers infectious substances which are capable of causing death or permanent physical disability if humans or animals are exposed to it.
Category B is known as UN3373 and is defined as any substances which are do not fit into Category A because exposure to it causes harm to a lesser extent in humans and animals.
Despite this difference, it is still mandatory to adhere to the packaging and labelling requirements for each category of specimens to ensure that the shipment and those handling it are safe.
We have created a classification chart for Division 6.2 substances here, which you can check if you are unsure which category your samples fall into.
Which government departments/bodies oversee UN infectious substance regulations?
In the United States, the transport of infectious substances is governed by the U.S. Department of Transportation (DOT) Hazardous Materials Regulations (HMR), 49 CFR Parts 171-180. The HMR applies to any infectious substance transported in commerce that is capable of posing a risk to health and safety. The HMR requirements must be met when transporting infectious substances by air, highway, rail or water.
Adherence to Category A and B compliance is also overseen by the bodies responsible for the transportation of dangerous goods via road and air: The European Agreement concerning the International Carriage of Dangerous Goods by Road (ADR) which covers road and rail transport, and the International Air Transport Association (IATA), which dictates global standards for airline safety, security, efficiency, and sustainability.
What packaging and labelling should I use?
IATA and ADR guidelines stipulate the packaging and labelling requirements that must be met when transporting Category A and Category B substances, all of which must be complied with to meet the standard. These regulations are known as Packing Instruction 650 (P650 or PI650).
The P650 instructions cover the required quality and construction of the packaging that must be used for transport and is split into guidelines for ADR and IATA. Each guideline has slightly different demands on packaging containing infectious substances, stipulating the following:
Packaging must consist of three components:
- A leak-proof primary receptacle(s) containing not more than 1 L / 1 kg (multiple primary receptacles must be individually wrapped or separated)
- Leak-proof secondary packaging
- A rigid outer packaging with one surface having minimum dimensions of 100 x 100 mm.
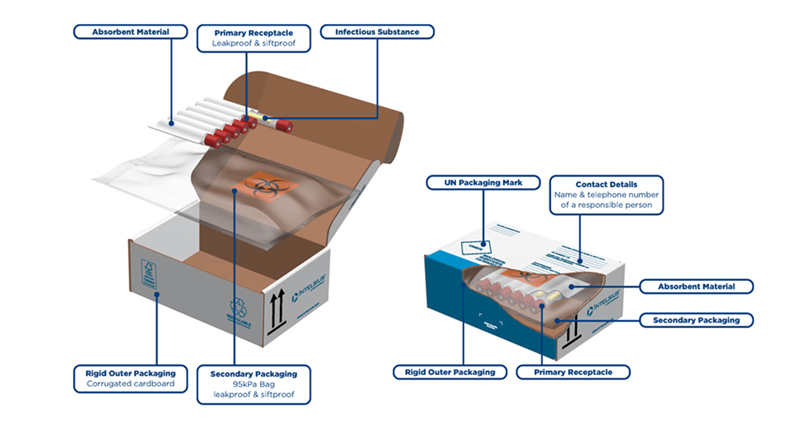
The packaging must be clearly labelled with either the UN2814/UN2900 or the UN3373 diamond and must have the proper shipping names for either Category. For Category A substances, the proper shipping names are:
For UN2814: Infectious substance, infecting humans
For UN2900: infectious substance, affecting animals only
Meanwhile the proper shipping name for UN 3733 is Biological Substance, Category B.
Some airlines prohibit the transport of Category B substances, and these must be consigned as Category A. Check with your airline or freight provider before shipping.
When transporting liquids, there must be sufficient absorbent between the primary and secondary packaging to absorb the entire liquid contents of the primary receptacle(s). There must be a maximum of 4 L per package.
When transporting solids, both the primary receptacle(s) and secondary packaging must be siftproof. Max 4 kg per package.
Do I need to prepare a Shipper’s Declaration?
According to P650, a Shipper’s Declaration is not needed for Category A and B shipments, however you would require other basic documentation, depending on which method of shipment your samples are being sent through.
For example, if transporting by air, you would need an Airway Bill which must indicate the name and contact number of the person responsible for the packaging, and the name and address of the shipper and consignee must be on each package.
Intelsius Category A and Category B Packaging Solutions
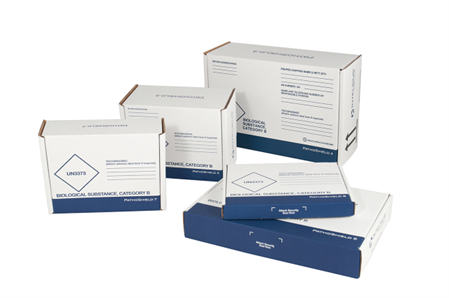
As a designer and manufacturer of Category A and B compliant sample transport solutions, Intelsius is well-positioned to help you with your dangerous goods packaging requirements. Intelsius has a range of Category A and B compliant packaging solutions for all your transport packaging needs. Our Pathopak range is both Category A and B compliant and adheres to IATA, ADR and CFR 49 (DOT) transport regulations and is suitable to ship a range of primaries including blood tubes, specimen containers, swabs, blood collection/transfusion bags and medical devices. This UN certified option is a strong and durable option that is not only highly protective but also cost-effective.

Our Pathoshield range is designed to cover a range of shipping solutions to carry a wide range of primaries including blood vials, swabs and sample containers. It is both Category A and B compliant and is ideal for use in all phases of clinical trials and to transport various samples and testing kits. For ease of use, each system is pre-printed with the required markings as per international transport regulations.
Get In Touch
Browse through our full range of Category A and B compliant sample transport solutions here. If you have any queries about transporting infectious substances or any questions about sample transport packaging, feel free to email cs@intelsius.com and our helpful representatives will be happy to be of assistance.

 Dry Ice Shippers
Dry Ice Shippers
 Pallet Shippers
Pallet Shippers
 Rental Service
Rental Service
 ORCA Solutions
ORCA Solutions
 Data Loggers
Data Loggers
 Brown Outer Shipper Boxes
Brown Outer Shipper Boxes
 Personal Medicine Carriers
Personal Medicine Carriers
 Category A Packaging
Category A Packaging
 Pathoshields
Pathoshields
 PathoPouch and PathoSeal
PathoPouch and PathoSeal
 Biotherm Extreme
Biotherm Extreme
 Biomailer
Biomailer
 Miscellaneous Components
Miscellaneous Components
 COVID-19 Sample Transport Packaging
COVID-19 Sample Transport Packaging
 Compliance Labelling
Compliance Labelling
 Cold Chain Guide
Cold Chain Guide
 UN3373 Sample Guide
UN3373 Sample Guide
 About Us
About Us
 Markets we Serve
Markets we Serve
 Services
Services
 Freight Charges
Freight Charges